Despite wearing bed sheets for every occasion, the Ancient Greeks were smart enough to conceive of atoms as building blocks of the universe. Without a whiff of technology at his disposal, Leucippus realised that all matter is composed of tiny bits which he named atomos, meaning indivisible.
This was a great starter hypothesis, even if the name turned out to be a total misnomer. Science has since brought us to the more robust conclusion that atoms are indeed divisible. What's more, all kinds of quantum quirks lurk within.
This is the story of how scientists decoded the atom using increasingly sophisticated tools, from cathode ray tubes to quantum equations. We'll see how each atomic model was incrementally refined until we reached today's quantum theory of atoms.
| Atomic Model | Features | |
 |
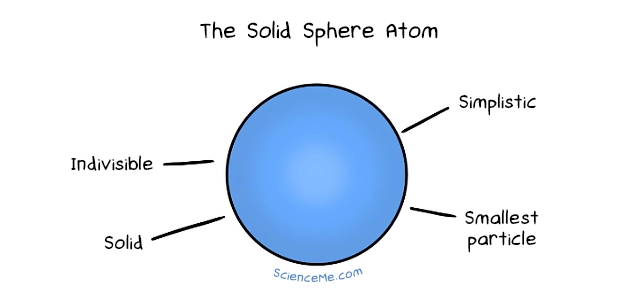
The Solid Sphere Atom (Dalton, 1803) | Atoms are simple, immutable, indivisible spheres |
 |
The Plum Pudding Atom (Thomson, 1904) | Electrons are embedded in positive solid spheres |
 |
The Nuclear Atom (Rutherford, 1911) | Atoms have a dense core with orbiting electrons |
 |
The Planetary Atom (Bohr, 1913) | Atoms have distinct energy levels and emit quanta |
 |
The Quantum Atom (Schrodinger, 1926) | Atoms have probabilistic electron clouds |
1. The Solid Sphere Atom
Our story begins in ancient Greece around 470 BCE, when Leucippus responded to philosophical claims regarding "what is" and "what is not". Specifically, he wondered how the universe came to exist when something had to come from nothing.
Leucippus said the universe is formed of two elements: the solid and the void. He imaged atoms as solid spheres that moved through the void, combining into new arrangements to create change in the macroscopic world. He even visualised the birth of stars: infinite atoms in constant motion, forming cosmic whorls which separate out the atoms by type until a membrane forms on the outer edge, trapping the centre atoms under increasing pressure until they catch fire.
His student, Democritus, ran with this idea, hypothesising that atoms differ in size, shape, and order. They bond using tiny hooks and barbs, he fancied, or repelled each another to prevent collisions. The resulting configurations amplify to produce macroscopic textures we see in the big world.
Not bad, right? That's where atomic theory sat for two millennia while civilisations rose and fell until, finally, in 1803, the English chemist John Dalton picked up the thread. Dalton mixed chemical elements and noticed they always reduce to the same ratios. "Leucippus was right!" he lectured Mrs Dalton in bed that night. "Atoms are solid indivisible spheres and definitely the smallest things ever."
Alas, Dalton was wrong on these counts. But his atomic theory was still valuable. Dalton formalised the idea of different species of atoms—the elements—each with their own unique mass and which combine to form molecules.
2. The Plum Pudding Atom
Atomic theory levelled up in 1897 when JJ Thomson discovered a new particle almost 2,000 times smaller than the smallest atom. Bazinga. It happened while he was experimenting with cathode rays: beams of charged particles which he called corpuscles emitted from atoms of gas inside a vacuum tube. This called for the Plum Pudding Model, which described atoms as spheres of positive matter studded with negatively charged corpuscles. The net effect was an overall neutrally-charged atom. Today, Thomson's corpuscles are known as electrons.
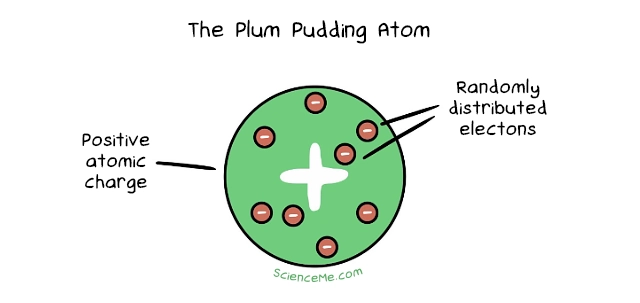
3. The Nuclear Atom
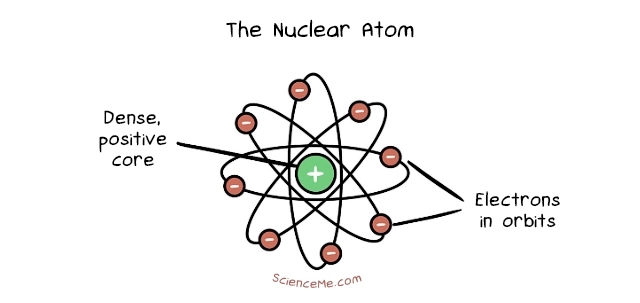
Soon after, in 1911, Ernest Rutherford thought it a good idea to shoot small atoms at bigger atoms to see how they'd interact. The incumbent atomic model suggested they'd bounce right off each other, and yet this is exactly what didn't happen. Most of the time, the small atoms shot right through the big atoms; around 1 in 20,000 times they bounced back.
Rutherford concluded that atoms are mostly empty space with dense core and smaller bodies in orbit, much like the Sun and its planets in the Solar System. He named the core the nucleus, which is packed with positively charged particles called protons and orbited by much smaller, negatively charged electrons.
4. The Planetary Atom
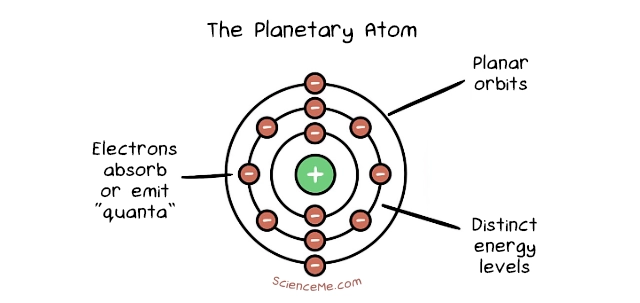
The Danish physicist, Niels Bohr, further adapted Rutherford's model by incorporating aspects of the newly emerging field of quantum theory. The differences come down to electron behaviour. In Bohr's Planetary Model, electrons orbit the nucleus in fixed energy shells which stabilise the atom. Electrons can jump between energy shells, absorbing or emitting electromagnetic radiation in the process. What's more, each energy shell can hold a maximum number of electrons, with the outermost shell's "fullness" determining the atom's chemical reactivity.
5. The Quantum Atom
This brings us to the present day model of atoms; one that's so fraught with peculiarity that most of us learned Bohr's Planetary model in school and stopped there. It's just easier to grapple with electrons flowing in predictable orbits around a nucleus—but this neglects the discovery of subshells and orbitals, quantum uncertainty, and why electrons don't simply collapse into the nucleus.
Cue Erwin Schrodinger who factored in the observation that electrons can behave like waves or particles, a phenomenon known as wave-particle duality. Schrodinger developed a complex mathematical equation to describe the behaviour of an electron in a hydrogen atom. The solutions to his wave equation, called wave functions, tell us the probability of finding the electron at any given point at any given moment.
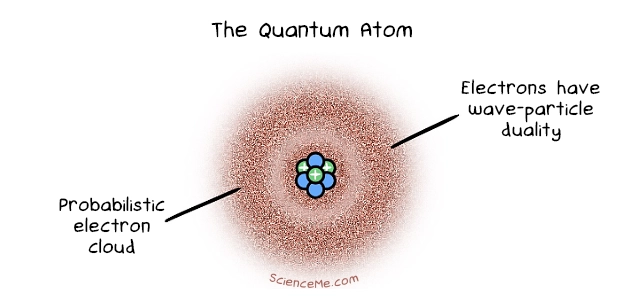
Schrodinger also taught us that electrons reside not in literal orbits or shells but in an electron cloud with variable densities. An electron is most likely to be found in high-density areas, but we can never know for sure due to Heisenberg's Uncertainty Principle. Remarkably, when this Quantum Model of Atoms was devised in 1926, science knew nothing of the existence of neutrons: neutrally charged particles that reside with protons in the nucleus. The credit for this goes to James Chadwick in 1932.
How Small Are Atoms?
Atoms are so small they're invisible to the naked eye. But how small are atoms really? On average, atoms have a radius of about 0.1 nanometres, or 0.0000001 millimetres. To put that another way, you could fit 5 million hydrogen atoms into a pin head.
That's quite the mindblower until you remember that atoms are 99.9% empty space; almost all of the mass of an atom is in the nucleus. If an atom were the size of a house, the nucleus would be the size of a speck of dust.
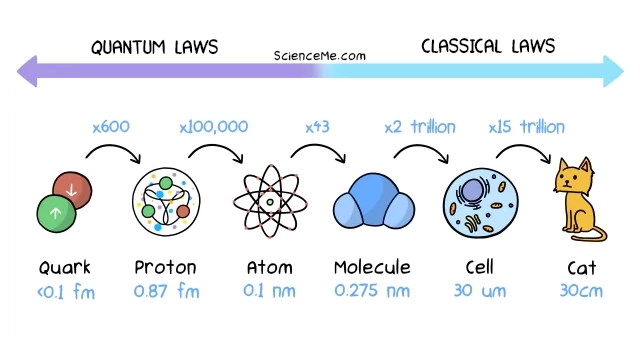
Philosophically, we might arrive at the conclusion that atoms aren't real at all. "Atom" is just the word Leucippus invented to describe what we now know is a system of electrons, protons, and neutrons. Like the Solar System, an atom is, more accurately, a Nuclear System—it's not a single "bit" of matter at all.
Are Quarks The Smallest Particles?
Hello? What's this quark business? If you were to smash open a proton you'd see three quarks burst out—at least theoretically. Quarks haven't been directly observed but are predictions based on some remarkable experiments:
Deep Inelastic Scattering. Bombard a proton with high-energy electrons and you'll see scatter patterns that strongly indicate the presence of point-like particles within.
Particle Collision Jets. In high-energy particle collisions at the Large Hadron Collider, jets of hadrons shoot out, thought to be made of quarks and gluons per the quark model.
Hadron Spectroscopy. The quark model also predicts the masses, charges, and other properties of hadrons, along with the existence of newly discovered particles like the J/psi meson.
So now we're really, pretty, almost, very sure that these elementary critters really are the smallest units of matter in the universe. Really.