Helium Could Save Our Planet
Huge helium reserves just beneath the Moon's surface could supply nuclear fusion reactors on Earth, offering a clean energy source for the 21st century.

On Earth, helium is a rare resource derived from limited reserves of natural gas trapped in the Earth's crust. It has a handful of advanced medical and industrial applications, not to mention party balloons.
However, the Earth-shattering potential for helium lies in nuclear fusion: a technology not yet fully developed but promises abundant clean energy for centuries to come. Scientists have long known that, despite its rarity on Earth, helium exists in abundance in space.
Vast reserves of the isotope helium-3 are trapped just under the surface of the Moon, giving it realistic potential for mining.
Helium's value as a nuclear power source pitches it at $3 billion per tonne. Even relatively small amounts shipped back to Earth could power entire countries year-round.
Combine the potential of lunar mining and nuclear fusion reactors and what have you got? An end to humanity's reliance on non-renewable fossil fuels, and protection for life on Earth from catastrophic climate change.
Such a transition is not only worth trillions in economic terms, but will pave the way for the next industrial and political superpower. The helium rush is on.
Helium in Space
The universe is predominantly made up hydrogen and helium. As the two lightest elements, they are the building blocks of all heavier elements.
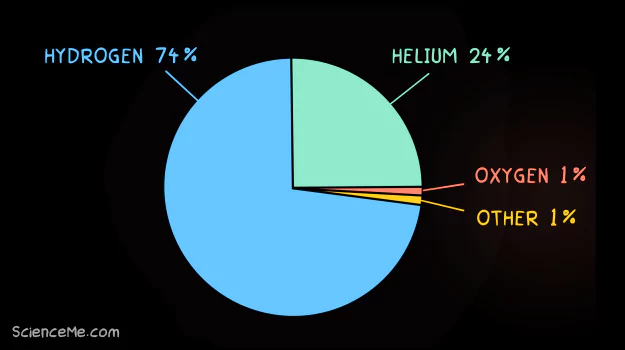
Hydrogen and helium are the most abundant elements in the universe by mass.
Hydrogen makes up three-quarters of the universe, including stars where it's burned into helium and later, heavier elements. Hydrogen is typically composed of one proton, one electron, and zero neutrons (see Atoms 101).
Stars are hot. Really hot. And this energy has all the hydrogen atoms shooting around—with the eventual outcome of colliding into one another. Usually when this happens, they break apart.
However, sometimes the collision simply causes one proton to lose its positive charge and convert to a neutron, thanks to the weak nuclear force. The result is an atom of deuterium: a stable isotope of hydrogen with one proton, one electron, and one neutron. The collision releases energy, ensuring our Sun stays hot, just as puny humans like it.
But we still don't have helium yet, so how is helium created? Picture the hydrogen atoms and deuterium isotopes whizzing around inside the star. Eventually these collide too, giving us an isotope of helium-3 (by adding another proton to deuterium).
Finally, when two atoms of helium-3 smack together, we get an atom of helium-4, plus a couple of hydrogen atoms as a nice bonus.
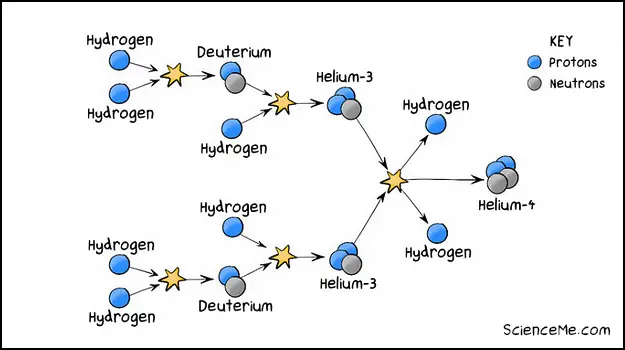
The nuclear fusion of hydrogen into helium
Hundreds of billions of stars are doing this in every galaxy, which is why there is a lot of helium hanging around in the cosmos. Not only is it rife inside stars, but it's also ejected on a massive scale and dispersed by solar winds, which is how it ends up in excess on the Moon.
Helium on Earth
But Earth's magnetosphere protects us from solar winds and the accompanying helium bombardment. So how is helium created on Earth?
It all comes down to rock. The radioactive decay of heavy elements such as uranium, thorium and radium emits alpha particles. And alpha particles, as Ernest Rutherford established, are essentially helium nuclei—just add electrons.
This process of decay, however, takes billions of years. Which is why helium is such a finite resource on our planet. We can and do mine the tiny helium pockets in the Earth's crust. However, when formed in the upper mantle, helium is released by volcanic activity and lost into the atmosphere.
From there, it's so light that it escapes Earth's gravity with ease. Thus, each time we deploy the stuff in party balloons or industrial applications, a little more is permanently lost to space.
The Discovery of Helium
Here's a cool story for you. Helium was discovered in stars back in the 1800s with the use of a spectrometer. This is a tool which diffracts light, enabling the observer to measure the optical properties of any substance, near or distant.
A spectrometer exploits the fact that electrons have specific energies which differ according to their orbital. When an electron relaxes to a lower orbital it emits light which shows up as an emission spectrum.
Conversely, when an electron is excited to a higher orbital it absorbs light which shows up on the absorption spectrum.
As you can see below, different elements have different optical fingerprints, allowing us to identify them even from vast distances across space.
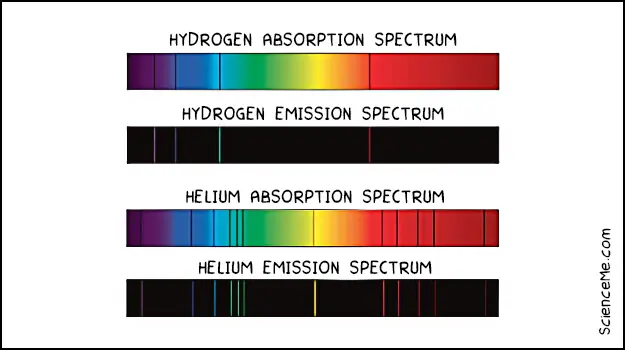
Comparison of the hydrogen and helium absorption and emission spectrums
Prior to 1868, astronomers observed the Sun during an eclipse and saw the spectral fingerprint of hydrogen, as well as a second unknown element, assumed to be sodium.
When the French astronomer, Pierre Janssen, made his own observations during an eclipse in India, he found the yellow line didn't match up with the wavelength of sodium. He went ahead an invented the spectrohelioscope, allowing him to take repeat measurements without the need for an eclipse, and confirmed the spectral fingerprint of helium—not sodium—coming from the Sun.
In the same year in England, the astronomer Joseph Lockyer was working on the very same incongruity, and came to the same conclusion that the second solar element was helium. It was a complete fluke that their letters, which stated identical findings, reached the French Academy of Sciences within a couple of hours of each other. The previously unknown element they discovered was named helium, after the Greek god of the Sun, Helios.
Fellow scientists showed a healthy scepticism about the findings and gave Lockyer and Janssen a hard time about it. But by 1881, the mysterious element helium was discovered in lava thrown up by an erupting Mount Vesuvius and the naysayers moved on hurriedly with their day.
What is Helium Used For?
Helium is mined from the Earth's crust for a range of applications. It's lighter than air—which makes it ideal for inflating air ships, blimps and balloons. This gives it a reputation for fun and games, however huffing on helium gas is ill-advised as it can cut off the oxygen supply to the brain, cause embolisms, and burst the lungs to cause haemorrhage, especially when taken direct from a pressurised tank.
Why does helium make your voice go high? Helium molecules have less mass than the oxygen and nitrogen in the air, so sound waves travel through them three times faster to give you the voice of a chipmunk. The opposite effect occurs when inhaling sulphur hexachloride, which is denser than air.
In deep sea diving, a mixture of 8:2 helium and oxygen is optimal for the pressurised conditions. And helium-neon gas lasers are used to scan barcodes in supermarket checkouts.
The Large Hadron Collider in Switzerland uses helium to cool and strengthen its electromagnets to -271°C. These magnets keep the beams of particles on track as they race around the collision course at close to the speed of light.
A new use for helium is a helium-ion microscope (HIM) that gives better image resolution than even a scanning electron microscope (SEM). It's also valuable as a recyclable cooling agent for super-magnets in MRI machines, and is used in space programs to displace liquid fuel in rocket tanks.
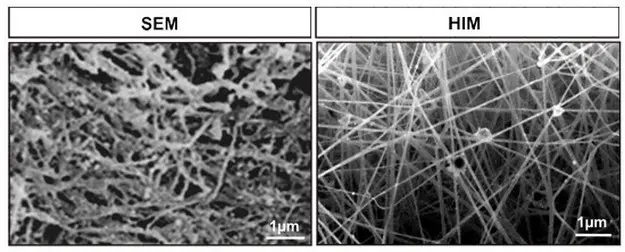
This fibrin matrix required coating with gold particles for viewing under SEM; HIM gives a better image resolution without any coating.
Is Helium Renewable?
We're due to run out of helium on Earth in the next two decades. This is problematic because many of the existing medical and industrial applications have no viable alternatives.
But here's the bright side. As you now know, the Moon lacks a protective magnetic field and has been bombarded with helium-3 solar winds for billions of years.
There are an estimated 1.1 million tonnes of helium in shallow pockets just under the surface of the Moon. And because helium is such a light element, that's a lot more than it sounds.
With sufficient investment, we can tap into the vast quantities of extra-terrestrial helium for use on Earth (and Mars when we get there). How will we access this resource and deliver it where we need it?
Besides existing applications, helium could be extremely valuable as a source of nuclear power. To appreciate this, we need to know the difference between nuclear fission vs nuclear fusion, and the benefits and risks of each technology...
Nuclear Fission vs Nuclear Fusion
All commercial nuclear power plants use nuclear fission to split apart heavy isotopes like uranium or plutonium. The reaction generates heat which is turned into electricity.
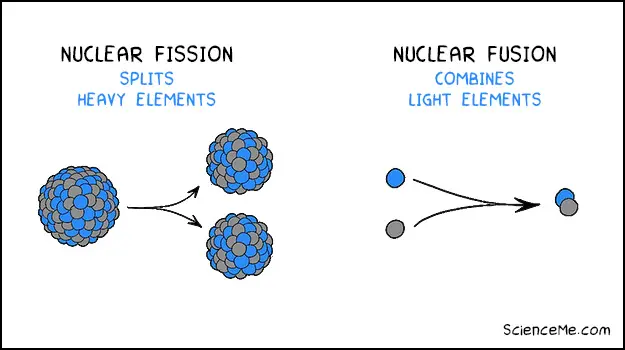
The difference between nuclear fission and nuclear fusion
However, nuclear fission comes with its drawbacks. Accidents like those at Three Mile Island (1979), Chernobyl (1986), and Fukushima (2011) reveal the serious and long term dangers of accidentally releasing large amounts of radiation into the environment.
What's more, the heavy elements required for nuclear fission are finite and non-renewable. They also produce radioactive nuclear waste as a by-product, even without the meltdowns.
In contrast, the dream of nuclear fusion has far greater appeal. And while we can't do it yet, it's a serious area of research among many nations, some of which have banded together for an international solution.
As opposed to splitting heavy atoms, fusion combines light atoms in high energy collisions, releasing large amounts of energy as a result. Not only is it more efficient than fission, but it's sustainable and produces virtually no radioactive waste.
Most nuclear research is geared toward the fusion of hydrogen isotopes (namely deuterium and radioactive tritium). The former is abundant in water and the latter is produced by the neutron bombardment of lithium. But the nuclear fusion scenario becomes even more attractive when you bring the non-radioactive isotope, helium-3, to the table.
Helium Fusion
There are a couple of problems we need to overcome before we can perform helium fusion for energy. First, getting two protons to fuse together (regardless of whether you're using hydrogen or helium isotopes) takes an awful lot of energy. It happens easily inside stars—but then they are giant balls of gas burning at millions of degrees Celsius.
Fortunately, physicists are making astonishing advances on this front. In 2016, Germany switched on its Wendelstein 7-X stellarator for the first time, creating temperatures up to 80,000,000°C to generate hydrogen plasma. Further development is ongoing to create an environment for higher temperatures and longer discharges, to reach the goal of net power generation, making nuclear fusion a reality.
Construction of the multi-billion-dollar International Thermonuclear Experimental Reactor (ITER) in France is on track for full fusion in 2035. ITER is a partnership of 35 countries which promises to be the first fusion device to create net energy.
Once we crack this nut, nuclear fusion with helium will be within our reach. That is, if you consider the Moon within our reach. And China certainly does.

The International Thermonuclear Experimental Reactor (ITER) Nuclear Fusion Reactor
Mining The Moon for Helium
Nuclear fusion is longer an impossible dream. Now the only problem with the helium plan is sourcing the actual helium itself.
On the Moon, helium-3 gas exists in vast quantities in the top few metres of the surface, making it a relatively easy operation to mine. The helium could be extracted by heating the lunar dust to 600°C before bringing it back to Earth, where it could power the entire planet for the next 200 years.
A fully-loaded space shuttle could carry 25 tonnes of helium, which is enough to power the US for one year. This puts its value at $3 billion per tonne, making the whole Moon mining escapade look very attractive in economic terms, if not logistical ones. And this is why helium could be our saviour.
China's Lunar Exploration Program, called Chang'e, is the most advanced project working towards mining the Moon, targeting both titanium and helium resources. Named for the Chinese Moon goddess, Chang'e runs an ongoing series of robotic missions that includes lunar orbiters, landers, rovers, and sample return spacecraft.
Chang'e intends to land crew on the Moon's south pole by 2030. It has also revealed plans to build a sustained human outpost for mining operations, which would arguably lend China economic supremacy if breakthroughs in nuclear fusion are secured.
Meanwhile, American, Russian, and Indian scientists have urged governments to plan for lunar mining. And while some governments have toyed with the notion, there's little tangible progress to date. Perhaps the (as yet) unfinished nuclear fusion capacity makes lunar mining too speculative to attract serious funding.
Nonetheless, as peak oil, resource depletion, and climate change rage on, there has never been more urgency to find new, alternative clean energy sources like helium.
All Articles

How Do Jellyfish Have Sex?
Jellies are ancient animals who mastered sexual reproduction long before us. Frankly, we're the ones odd-balling it with our penises, vaginas, and miserable childbirth.

15 Popular Science Books
Reading is like getting into another person's brain pool and swimming around in their knowledge. There is only one rule. DON'T PEE IN THE POOL.

Best Lex Fridman Podcasts
Lex Fridman hosts a popular science podcast in which he digs into the minds of world-class experts and extracts intellectual treasures.
How Does ChatGPT Work?
ChatGPT is a large language model, or LLM, that can debate moral philosophy, write short stories, and help you do your taxes.
The Evolution of SARS-CoV-2
As the youngest of the coronavirus family, SARS-CoV-2 did his parents proud. Here's what we know about its genetic relatives and how it evolves by antigenic drift.

The Life of Isaac Asimov
Asimov described his short story, Nightfall, as an archetypal social science fiction, moving away from gadgets and toward exploration of the human condition.

How Does DNA Work?
DNA isn't just a blueprint for foetal growth—you're expressing DNA right now to produce life-sustaining proteins like insulin, cortisol, and oxytocin.

Nanomedicine is Here
Nanomedicine is shaping our lives, with 50+ nanomedicines already in general use, including lipid nanoparticles (LNPs) in mRNA vaccines.
12 Rules for Life Illustrated
12 Rules for Life is a psychological manifesto from a man who's making a giant existential omelette and breaking more than a few eggs in the process.
Can We Live on Mars?
What are the challenges of colonising Mars? A look at terraforming and artificial life support to make Mars habitable for humans.





